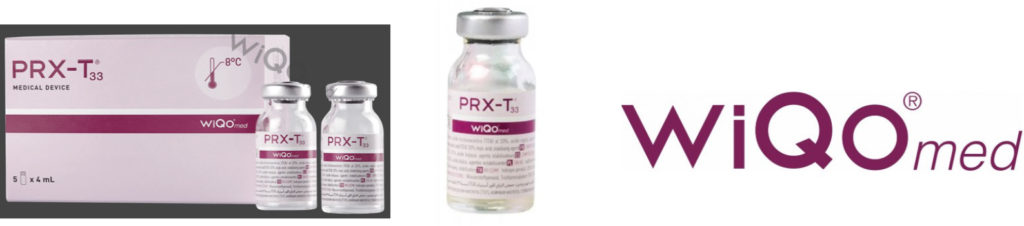
Unauthorized Dealers
WARNING: Currently, the only legal/authorized distributors of PRX Derm Perfexion (formerly PRX-T33) in the US are LoveBeautyPro.com and ModernMedicalAesthetics.com. The only approved US product that is safe is the new Derm Perfexion packaging. This new packaging strategy was created by a partnership between LoveBeautyPro and WiQo to help providers and practices identify the correct and official product.
PRX supplied outside the authorized distributors is not approved for use in the US and is grey market product. It is unethical and potentially dangerous to use this product on patients as this unauthorized product may be counterfeit and not properly stored or shipped, which would adversely affect the efficacy of the product and the outcome for your patients.
WiQo as the manufacturer does not support or guarantee the quality or safety of use of products purchased from unauthorized grey market sources. Furthermore, it may create adverse reactions which we would not be able to provide any assistance or guidance for use or questions on this product. GPQ (WiQo parent company) is registered with the US FDA in the VCRP as a cosmetic manufacturing establishment. PRX Derm Perfexion (formerly PRX-T33) is classified and marketed according to the current FDA recommendations. These classifications and protections do not apply to grey marketing product. If a provider/practice buys diverted illegal product they will not be covered by the manufacturer or their insurance in the event there is an adverse reaction.
Insurance companies consider the use of counterfeit product medical negligence.
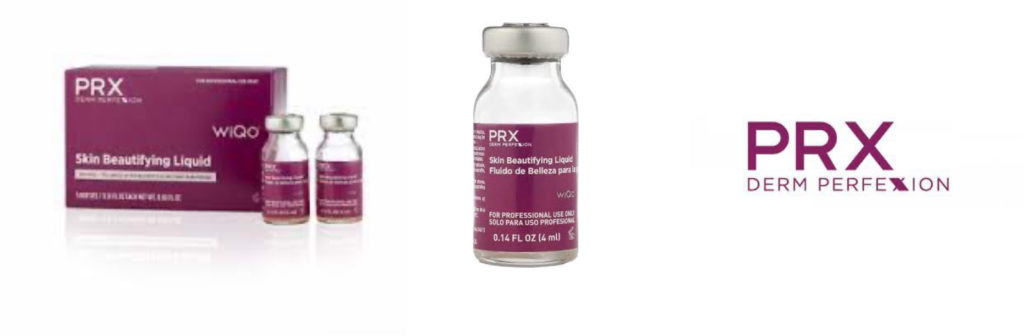
OFFICIAL & AUTHENTIC PRX Derm Perfexion packaging. Updated in March 2022. This is what your packaging should look like. ONLY order through US Distributors!
When you order from an authorized US Distributor, your product should NOT look like this!
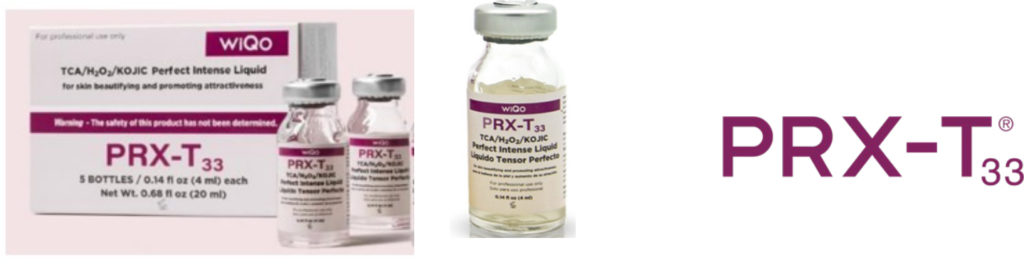
Illegal in the US! Be compliant to US Regulations. Stay away from product that looks like this! Avoid adverse events, legal and insurance issues, and unsatisfactory patient outcomes. Insurance companies consider the use of counterfeit product medical negligence. Do not use!